Electrospray Mass Spectrometry Laboratory
Department of Biochemistry

Laboratory leader: Dr André Venter
Introduction to Electrospray Mass Spectrometry
In recent years mass spectrometry (MS) has been made more accessible to the Biological Sciences by the development of non-fragmental ionisation methods. One of these methods, Electrospray ionisation (EI) is becoming increasingly popular and is currently employed in the VG Quattro mass spectrometer operated in our Department (Figure 1). This instrument forms part of the Central Analytical Facilities of the University of Stellenbosch.
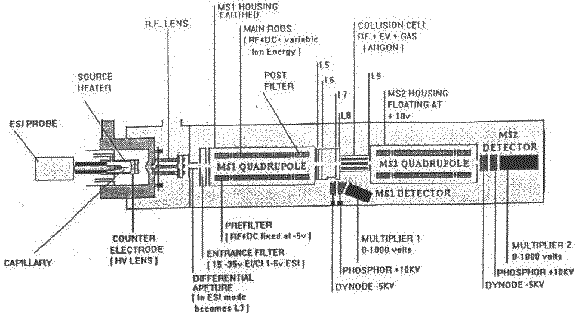
Figure 1. Schematic representation of the VG Quattro mass spectrometer with electrospray ionisation source.
Electrospray ionisation takes place in the inlet and source sections of the instrument (Figure 2). A mixture of an aqueous solution of a sample and an organic solvent is pumped through a hollow needle held at a high potential (3-4kV). The stream of liquid issuing at 5-300Ál/ml is broken up into highly charged droplets of ca. 1 micron to form the "electrospray". Dry nitrogen gas is also introduced through a capillary to enhance nebulisation (Figure 2). This occurs at atmospheric pressure.
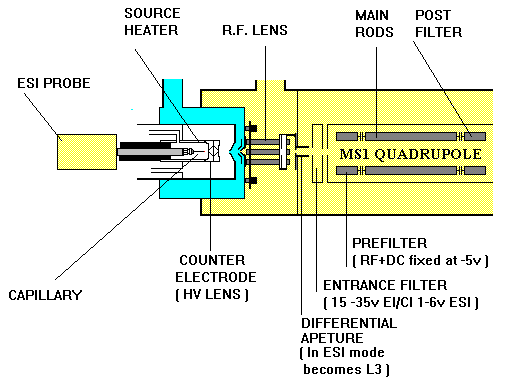
Figure 2. Schematic representation of the electrospray ionisation source.
The charged droplets are accelerated in an electric field towards the analyser section of the instrument held at a high vacuum. Through the combined effects of drying gas and vacuum the solvent in the droplet starts to evaporate giving rise to smaller, increasingly unstable droplets from which surface ions are liberated into the vacuum (Figure 3). The desolvated ions pass through a sample cone and skimmer lenses and after focusing by the RF lens, into the high vacuum region of the quadropole analysers where they are separated according to mass and detected by photomultiplier detectors (Figure 1).
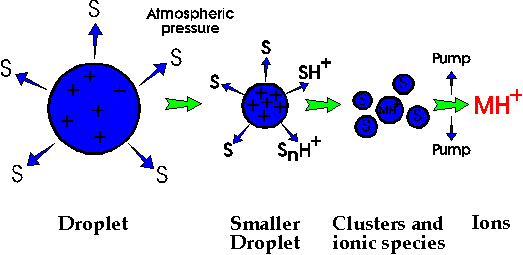
Figure 3. The desolvasion process taking place in the electrospray ionisation source.
Analyses of Biopolymers
This mild ionisation method makes electrospray mass spectrometry (ESMS) one of the methods of choice for the analysis of large and labile biopolymers such as oligonucleotides, proteins, peptides and sugars. During ionisation, large biomolecules are normally multiply charged. For protein studies in positive mode, this amounts to about one charge per 1000 daltons of protein. These charges are normally situated on the amino groups of the basic amino acid residues and the terminal amino group. The number of charges varies somewhat with the specific amino acid content of the protein and the pH of the spraying solution.
The typical spectrum of a protein reveals a number of peaks, usually centre around a m/z range of 1000 (Figure 4). Each of these peaks represents an ion specie with one charge more than the adjacent peak to its right.
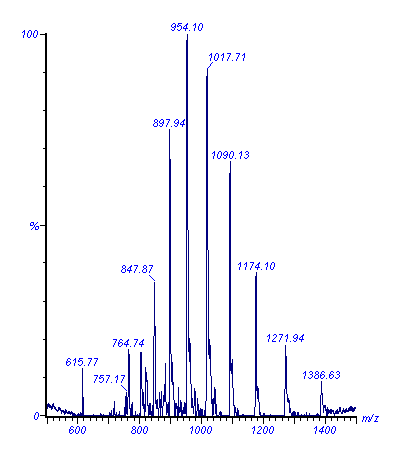
Figure 4. ESMS spectrum of sheep cytochrome b5
Using this data computational correlation can determine the specific charge state of each ion peak which can then be converted to a so-called transformed spectrum showing the actual mass of the protein (Figure 5). Because several peaks on the m/z scale are used during the computation, the accuracy of the molecular weight calculation is very high.
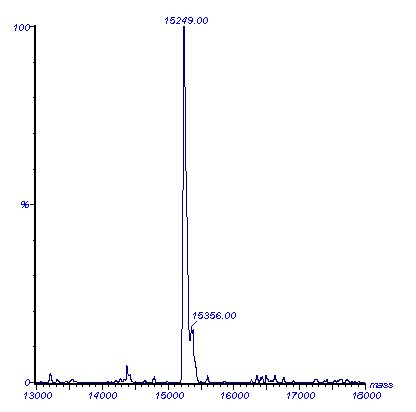
Figure 5. The molecular mass of sheep cytochrome b5, calculated by the program MaxEnt, from the ESMS spectrum shown in Figure 4.
The Fragmentation Option
The VG Quattro is a triple quadropole analyser, with two analysing quadropoles in tandem, separated by a non-analysing quadropole between - by convention, a QqQ instrument (Figure 1). The non-analysing quadropole serves as a fragmentation cell where collision induced fragmentation of ions, selected by the first quadropole, can be effected. This is brought about by the introduction of an inert gas such as Argon into the fragmentation cell. The resultant fragments (product ions) can then be mass analysed by the second quadropole to give more structural information of the analyte, as demonstrated by Figure 6.
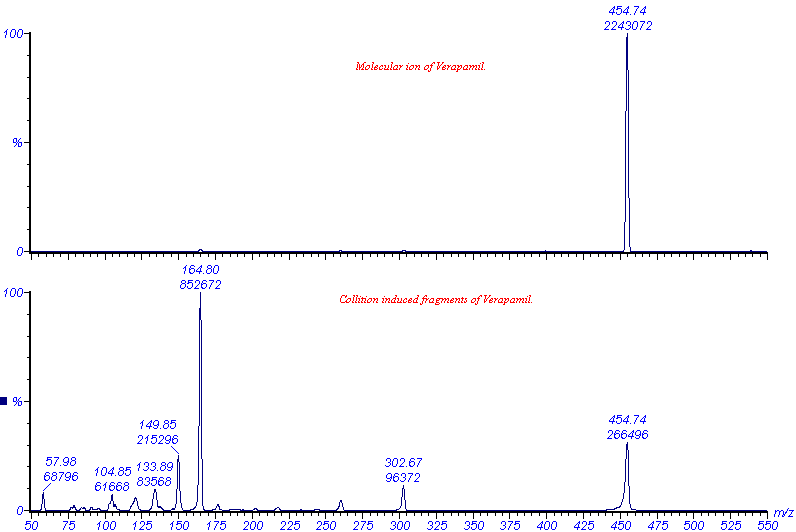
Figure 6. ES-MS analyses of Verapamil: the molecular ion was selected by the first analyser, fragmented by collision with argon and mass analysed by the second analyser.
Coupling of ESMS to Separation Techniques
Because the analytes introduced into the ESMS are dissolved in an aqueous organic solvent such as 50% acetonitrile, the ESMS is compatible with solvent based separation techniques such as high performance liquid chromatography (HPLC) and capillary electrophoresis (CE). In contrast to gas chromatography coupled to MS, HPLC/ESMS requires no derivatisation of the analyte and therefor time and effort is saved in the preparation of the sample for analyses. HPLC/ESMS and CE/ESMS are fast becoming important tools in the analyses and tracing of extremely small quantities (femtomolar concentrations) of analytes as diverse as serum proteins and pesticides.
All the above can be considered as a very brief introduction to the multi-faceted and fascinating field of ESMS. This facility is considered to be an important part of the infrastructure of our Department, and has already made valuable contributions to research and service, not only in our Department or University, but also to the Industry and the Scientific Community country wide. We welcome contact from anyone who wants to make use of the facility or who is in any way interested in the technique.
Please contact Dr. André Venter by e-mail at the following address: aventer@sun.ac.za