 |
|
Research: Inhibitors of cofactor utilization
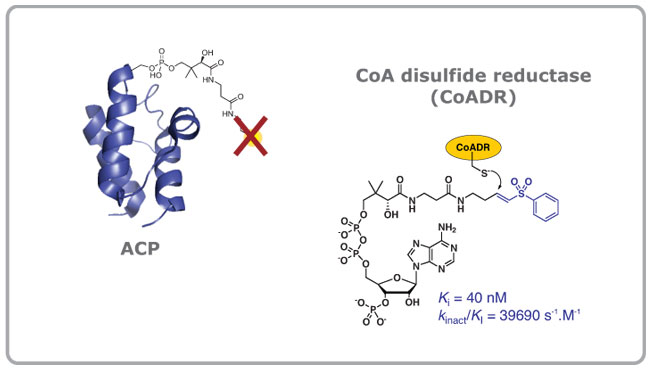
The central role of CoA in energy and fatty acid metabolism means that CoA analogues (or their precursors) in which CoA’s thiol moiety has been replaced may act as antimetabolites. This has been shown to be the case for ethyl-dethia-CoA, which has the thiol group exchanged for a propyl group, and which acts as an inhibitor since its modified pantetheine moiety is transferred to the acyl carrier protein (ACP). This inactivates the ACP, and renders it useless in fatty acid metabolism. We are currently exploring the scope of a class of compounds that act as precursors to such inhibitors, called the pantothenamide, to determine their SAR and utility as bacteriocidal agents.
At the same time we are also interested in the role of unique and specific low molecular weight thiols and the flavin-dependent disulphide reductase enzymes with which they are partnered in maintaining the redox balance in pathogenic organisms such as S. aureus and M. tuberculosis. In recent studies we developed the first known inhibitors of the CoA disulphide reductase of S. aureus – these were based on Michael-acceptor containing CoA analogues which irreversibly modified a key active site cysteine residue in the enzyme. We also demonstrated that these analogues may be prepared by the CoA biosynthetic enzymes acting on the corresponding pantothenamide precursors, indicating that the pantothenamides may act as prodrugs in whole cells tests. Studies that expand on this work are currently underway.
|
|

