|
|
|
|
|
Research Emphasis: Physical, Inorganic, Organic, Materials, Structure-Property Relationships, Solid-State Structure, Gas Storage/Separation,
Crystal Engineering, Method Development
|
|
|
|
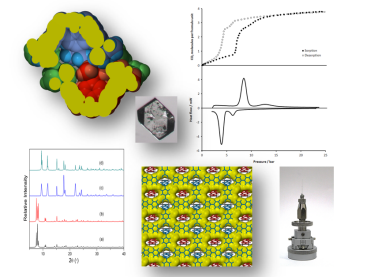
For centuries, conventional porous materials such as zeolites and activated carbons have been used for many important applications such as catalysis, air and water purification, chemical separation, molecular sequestration, nuclear waste treatment, etc. Over the past two decades there has been an explosion of research aimed at developing new types of porous materials based on molecular solids, metal-organic frameworks and covalent organic frameworks. It is anticipated that these new designer solids will rival and, ultimately, far surpass the useful properties of conventional porous materials. Since “Nature abhors a vacuum”, the creation of molecular space in the crystalline solid state involves a number of interesting challenges; there are now many groups around the world that focus almost exclusively on the design, assembly and preliminary structural characterisation of new porous crystals. However, comprehensive follow-up studies of the properties of these materials are seldom undertaken. The primary focus of the Barbour group is to develop the tools to understand counterintuitive behaviour (particularly the dynamics of structural flexibility) of porous materials. In addition to detailed crystal structure analyses, we carry out physico-chemical measurements of the kinetics and thermodynamics of sorption processes. We also actively develop new devices and protocols that we apply routinely to our own research: these include an environmental gas cell for in-situ structural analysis of gas-loaded crystals (by means of single-crystal and powder X-ray diffraction), and pressure-gradient differential scanning calorimetry.
|
|
 |
ANOMALOUS THERMAL EXPANSION
|
|
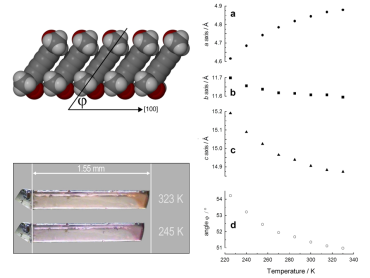
Most materials expand in all three dimensions when heated, a phenomenon known as positive thermal expansion (PTE); longitudinal thermal vibrations of the constituent atoms increase in magnitude and frequency, and the molecules therefore move away from their neighbours. However, for some solids thermal expansion occurs anisotropically, that is, with highly disparate linear expansion coefficients along the three directions. In rare cases, structural mechanisms exist that cause negative thermal expansion along one or more directions. This area of structural chemistry is still relatively unexplored and we are actively involved in establishing the structure-property relationships that influence anomalous thermal expansion.
|
|
 |
|
|
|
The group maintains a well-equipped workshop (2 lathes, a milling machine, TIG welder, electronics workstation, gas reticulation station stocked with Swagelok components, etc.) for the development and modification of instrumentation. We construct custom sorption equipment and automated gas delivery systems. We have also developed an environmental gas cell for X-ray diffraction analysis (both single-crystal and powder) of samples under gas pressure (0 - 80 bar). The software that controls these devices is developed in-house. The ability to develop or modify our own equipment allows us to carry out unique types of experiments.
|
|
 |
|
© 2015 Department of Chemistry and Polymer Science, University of Stellenbosch, Stellenbosch, 7600, South Africa, Tel. +27 (0)21 808 3335, Email: ljb@sun.ac.za
|







